Abstract
Background: There are significant disparities in cancer care and outcomes. Many new drugs have been recently approved for hematologic malignancies through randomized clinical trials (RCTs). Equitable population participation in RCTs is important to ensure proper representation of the populations suffering from the malignancies being targeted. It is uncertain whether patients enrolled in clinical trials represent the demographics of a given malignancy. In this study, we evaluate the extent to which trials match disease burden and how trial methods and results differ across racial/ethnicity/minority disparities in participation of clinical trials.
Methods: We performed a retrospective review of RCTs published from 2017 to May 2021 that led to Food and Drug Administration (FDA) approval of hematological malignancy drugs (Leukemias, Multiple Myeloma (MM), Myelodysplastic Syndrome (MDS), and Myeloproliferative Neoplasm (MPN)). We excluded drugs approved for Amyloidosis, pediatric studies, Blastic Plasmacytoid Dendritic Cell Neoplasm, and Erdheim-Chester Disease (Non-Langerhans Histiocytosis). The drugs investigated were selected using FDA Databases, 'Oncology (Cancer) / Hematologic Malignancies Approval Notifications' and 'Novel Drug Approvals'. A Pubmed search was conducted using the drug name and/or clinical trial number as key terms to identify manuscripts related to the approved drugs. The manuscripts were verified with respective FDA drug approval announcement to ensure that this was the appropriate study. 34 drugs were found using the inclusion/exclusion criteria, only 12 drugs had primary manuscripts with demographics including race. Data was then extracted from NIH Surveillance, Epidemiology, and End Results Program (SEER) for prevalence on 1/2018 by race for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), MM, chronic myeloid leukemia (CML), and MDS that the 12 drugs were approved for. SEER data is collected over 13 US locations.
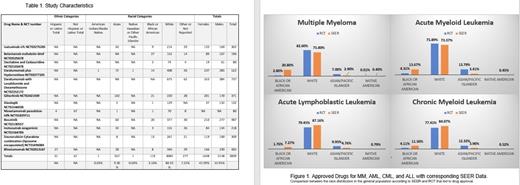
Results: The study characteristics for the 12 drugs and demographics are found in Table 1. These included 4 drugs approved for MM, 3 for AML, 2 for ALL, and one each for CML, MDS, and hairy cell leukemia with a total of 3839 patients. The study with the largest sample included 176 sites in 14 countries, and the smallest 15 sites in 2 countries. MM studies enrolled 1757 patients, with 123 (7%) Asian/Pacific Islander patients, 50 (2.8%) Black/African American, 1452 (82.6%) Whites (including Hispanics), 1(<0.1%) Native American patient, and 131 (7.4%) did not report. AML studies enrolled 812, with 112 (13.8) were Asian/Pacific Islander, 35 (4.3%) Black/African American, 616 (75.9%) Whites (including Hispanics), and 49 (6.0%) did not report. ALL studies enrolled 623, with 62 (10%) Asian/Pacific Islander, 11(1.8%) Black/African American, 495 (79.5%) Whites (including Hispanics), and 55 (8.8%) did not report. The one MDS study enrolled 80, with 0 Asian/Pacific Islander, 2 (2.5%) Black/African American, 74 (92.5%) Whites (including Hispanics), and 4 (5.0%) did not report. The one CML study enrolled 487, with 60 (12.32%) Asian/Pacific Islander, 20 (4.11%) Black/African American, 377 (77.41%) Whites (including Hispanics), and 30 (6.16%) did not report. The one hairy cell leukemia enrolled 80: with 1 (1.3%) Asian/Pacific Islander, 1 (1.3%) Black/African American, 70 (87.5%) Whites (including Hispanics), and 8 (10%) did not report. Two studies reported ethnicity, Hispanics and Non-Hispanics. Sex was not differentiated by race in the studies. All but one study showed sex overall, with 1648 Women and 2148 Men across the 12 studies. Figure 1. shows in all diseases that Black/African-Americans are underrepresented. African Americans had an 86.5% (MM), 68.% (AML), 75.8% (ALL), and 64.2% (CML) percent lower representation compared to SEER demographics. In contrast, Asian/Pacific Islanders had a percent increase from the SEER population by 58.6% (MM), 75.3% (AML), 52.1% (ALL), and 68.3% (CML). There were significant differences in all racial categories for the four disease with the exception of Native American representation in AML and CML, and White representation in ALL and CML when compared using Z-statistics.
Conclusion: The misrepresentation of minorities in pivotal clinical trials may lead to results that may not fully translate to such populations. These disparities in enrollment should be corrected in future studies.
Cortes: Novartis: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Bristol Myers Squibb, Daiichi Sankyo, Jazz Pharmaceuticals, Astellas, Novartis, Pfizer, Takeda, BioPath Holdings, Incyte: Consultancy, Research Funding; Bio-Path Holdings, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.